
Published on the 21st December 2023 by ANSTO Staff
Key Points
- A special zinc additive dialkyl dithiophosphate (ZDDP) enhanced the performance of thin lithium metal strips for lithium-ion batteries
- A cycle lifetime of up to 2800 hours was maintained even at a high area capacity
- The team used X-ray absorption spectroscopy to characterise the anode samples
A research team led by scientists from Central South University, Changsha, Hunan, China have used the Australian Synchrotron in developing a novel strategy for the scalable production of high-performance, thin, and free-standing lithium anodes for lithium-ion batteries with enhanced cycling stability and electrochemical properties.
The work was undertaken to meet the growing demand for high-performance lithium-ion batteries. Solid-state lithium metal has a high energy density and high capacity theoretically, making it an ideal replacement for traditional graphite anodes.
In a paper published in Nature Communications, the team reported that a special zinc additive dialkyl dithiophosphate (ZDDP) enhanced the performance of thin lithium metal strips.
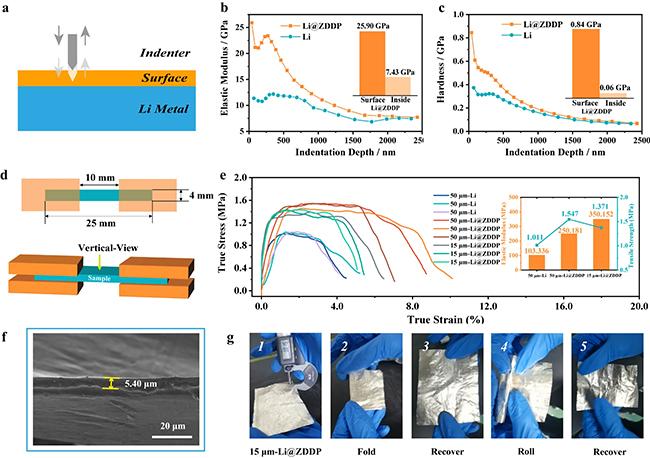
The research demonstrated that the additive increased hardness at the interface, prevented structural degradation (growth of lithium dendrites), controlled the deposition of lithium during plating/stripping, and the lithium anode could be plated and stripped faster than other materials.
The team produced thin lithium strips with thicknesses ranging from 5 to 50 micrometres, with better mechanical strength, electrochemical performance and impressive cycling stability compared to untreated lithium strips.
A cycle lifetime of up to 2800 hours was maintained even at a high area capacity. Additionally, a symmetrical cell based on ultrathin lithium strips with 15 micrometres thickness lasted for more than 800 hours.
The study also included a full cell configuration using LiFePO4(LFP) and ZDDP-coated lithium, showing excellent cycling life with over 83.2% capacity retention after 350 cycles. In comparison, a cell without ZDDP degraded rapidly.
The improved electrochemical characteristics of the ZDDP-coated lithium anode were attributed to the creation of a high-strength artificial solid electrolyte interface (SEI) layer with a high affinity for lithium.
Instrument scientist Dr Bernt Johannessen said this was an example of innovative work in the development of ultra-thin lithium, only microns thick that is manufactured for solid-state batteries.
For this study, a zinc-based oil was developed and used during the production process, in which the lithium is rolled out thinner and thinner, similar to how you roll dough through a pasta machine.
Samples were mailed to the synchrotron, and Dr Johannessen undertook the measurements of the lithium anodes using the X-ray absorption spectroscopy beamline, which has proved particularly useful in investigating energy materials and catalysis.
So much so that the beamline has produced nearly twice the number of publications through 2023 as compared to the previous year.
“On that note, we owe our user community a debt of gratitude; they are being wonderfully productive and taking full advantage of recent developments, such as fast scanning techniques, at the beamline,” said Dr Johannessen.
Dr Zhibin Wu, the second author, was a recipient of the AINSE Postgraduate Research Award recipient and studied under the co-supervision of Dr Bernt Johannessen during his PhD at the University of Wollongong.
Dr Johannessen maintains connections with many of his former students, including Dr Wu, and has a decade-long association with the University of Wollongong, recently acknowledged through a Fellowship.