
Published on the 11th April 2017 by ANSTO Staff
Small angle neutron scattering (SANS) at the Australian Centre for Neutron Scattering has confirmed that the strength of a type of supramolecular hydrogel can be increased by heating.
Hydrogelators, which have a stacked fibrous network structure, can potentially be used in bioengineering, pharmaceutical technologies, environmental remediation, catalytic processes and personal care products.
In collaborative research published in Soft Matter earlier this year, a group of Australian investigators led by Curtin University and the University of Western Australia, also confirmed that the selection of electrolyte plays a key role in determining the strength of a hydrogel formed by calixarene (bound with the amino acid proline).
Upon heating to 30° C, calixarene hydrogels formed with the electrolyte magnesium chloride increased in strength and maintained increased stiffness upon cooling.
The compound exhibited significant hysteresis (a memory of the initial structure).
The investigators pointed out that this unusual, reproducible behaviour did not result in a loss of water upon heating.
Because the gel stiffness got smaller over repeated annealing, it suggested a dynamic rearrangement process was occurring.
Atomic force microscopy imaging at the University of Western Australia revealed that new larger, straighter fibres formed at 25 – 30° C, possibly supporting the formation of a more robust gel while smaller fibres appeared to dissolve.
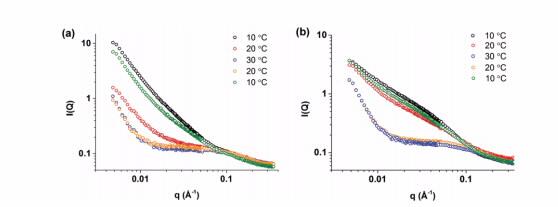
Co-author Instrument scientist Chris Garvey authors confirmed the model of what was happening to the gel structure following annealing over the temperature range 10-30° C using SANS on QUOKKA.
 |
| Scattering profiles obtained from SANS measurements for gels of LiCl• 1 (a) and MgCl2•1 (b) upon cycling the temperature from 10–30–10° C |
“SANS is the technique of choice for gelators because can determine how molecules behave in an ensemble while a parameter is changing,” said Garvey.
Scattering data indicated a change from uniform rod-like structures to a more complex system.
“In this study, the SANS data was consistent with the structural model and scattering curve calculations,” said Garvey.
The authors report that in the MgCl2 hydrogel this represents a change from the initial configuration of long, flexible fibres to short, straight fibres upon heating, which become long, straight fibres when the system is cooled.
The hydrogel formed with the electrolyte lithium chloride responded differently to annealing.
“One of my co-authors is interested in tissue scaffolding as part of dementia research but there are a great many potential applications for low molecular weight gelators,” said Garvey.
"The study was undertaken because there are still aspects of their behaviour that need investigation, such as how gel structure evolve over time and how to ensure the stability of load bearing structures."
The University of New South Wales and the ARC Centre for Convergent Bio-Nano Science and Technology also contributed to the study.
http://pubs.rsc.org/en/content/articlepdf/2017/sm/c6sm02431a