
Published on the 28th July 2015 by ANSTO Staff
Scientists from the University of New South Wales, the Czech Technical University in Prague and the University of Sydney, along with ANSTO researchers from the chemical deuteration team at the National Deuteration Facility, have shown that the replacement of hydrogen with deuterium in the emitting species of photovoltaic devices enhances upconversion efficiency by reducing the first-order decay rate constant.
Led by the group of Professor Tim Schmidt at UNSW, the researchers anticipate that the observations are applicable to a range of emitting species, and provide the opportunity to improve the device efficiency of organic light emitting diodes (OLEDs). This represents the first work published by the National Deuteration Facility which exploits the kinetic isotope effect to enhance materials using deuteration.
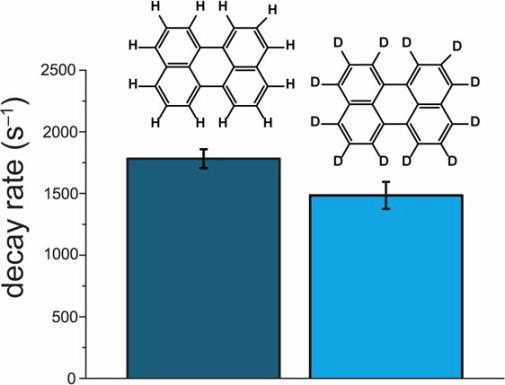
 Deuteration of perylene reduced the first-order decay rate constant by 16 ± 9%, which increased the upconversion efficiency by 45 ± 21%. Small polycyclic aromatic hydrocarbons such as perylene are often employed as the emitter molecules in upconversion OLED devices. In this study, an increase of 45% of the relative upconversion efficiency was observed when deuterated perylene was used as the emitter species in place of the hydrogen isotopologue. Analysis of two systems (one using perylene-h and the other perylene-d) via action spectroscopy and time-resolved photoluminescence spectroscopy allowed the scientists to isolate the effect of changing molecular vibrations on the first-order decay rate constant by replacing hydrogen with deuterium. While other chemical substitutions (replacement of hydrogen with fluorine or a methyl group, for instance) could be used to alter molecular vibrations in an attempt to improve emitter efficiency, these substitutions affect the electronics and geometry of the molecule in addition to altering vibrational modes. These changes might have complex impacts on other important emitter parameters and render any efficiency gains unpredictable. The isotopic substitution of deuterium for hydrogen decreases the energies of vibrational modes, but the geometry and electronics of the molecule are maintained, allowing the effect of changing molecular vibrations to be studied in isolation. Reference: A. Danos, R. W. MacQueen, Y. Y. Cheng, M. Dvořák, T. A. Darwish, D. R. McCamey and T. W. Schmidt, J. Phys. Chem. Lett., 2015, 6, pp 3061–3066 |