
Published on the 14th February 2017 by ANSTO Staff
Experiments at ANSTO have provided supporting evidence of unexpected enhancement of water solubility of biomolecules in an aqueous solution of divalent transition-metal cations.
The discovery is significant for understanding physical, biological and chemical processes involved in drug design, functions and synthesis of macromolecules and may even provide clues in the prevention of Alzheimer’s disease.

Fundamentally, the findings may potentially change the traditional view of the interactions between biomolecules with metal cations.
This research outcome, recently published in Physical Review Letters, is part of an international collaboration between ANSTO’s Australian Centre for Neutron Scattering (ACNS) and the research group for interfacial water at Shanghai Institute of Applied Physics (SIAP), Chinese Academy of Sciences, Shanghai, China.
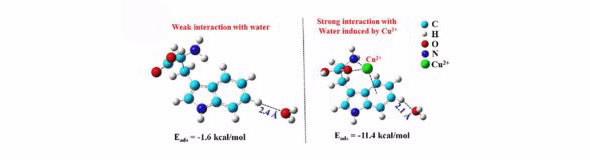
Generally, in many multivalent transition-metal ions, such as Cu2+, Pt2+, Pd2+, and Co3+ in solution, the solubility of aromatic amino acids decreases significantly because most of them will form complex precipitates with the ions, as documented in standard chemistry and biochemistry reference works.
However, considerably increased solubility of tryptophan (Trp) in a CuCl2 aqueous solution has been observed experimentally and predicted theoretically by the research group led by Professor Haiping Fang at SIAP.
This unusual phenomenon can only be observed under a special condition of a high local concentration of Cu2+ at the surface of Trp.
Fundamentally, it is attributed to the strong interaction between Cu2+ and the aromatic ring in Trp, referred to as the cation-p interaction.
Co-authors Dr Dehong Yu and Dr Richard Mole measured quasi-elastic neutron scattering (QENS) on the Pelican instrument at ACNS. The experiment provides direct evidence of the strong cation-interaction responsible for the enhanced solubility of Trp in a CuCl2 aqueous solution.
QENS exploits small energy exchanges between the diffusing particles and scattered neutrons, which is directly related to the diffusive processes taking place in the system. The self-diffusion coefficient of the system can be obtained from the QENS measurement.
The significant difference in diffusion coefficients between tryptophan with and without Cu2+ suggested that the tryptophan complex containing Cu2+ moved much more slowly than the tryptophan without Cu2+.
This observation supports the theoretical prediction of the enhancement of water affinity due to the presence of Cu2+.
“In this experiment, we make full use of the QENS capability of the Pelican instrument and the high sensitivity of neutrons to hydrogen atoms to study Trp dynamics under different environments, as the QENS signal from the entire system is dominated by the hydrogen atoms in tryptophan,” said Yu.