Published on the 26th June 2024 by ANSTO Staff
In late 2023, the ARC Centre of Excellence (CoE) for Green Electrochemical Transformation of Carbon Dioxide – GETCO2 – officially kicked off a $45M, 7-year research collaboration funded by the ARC, university, industry and government partners. This collaboration will support innovative approaches to carbon capture.
GETCO2 seeks to position Australia as a global leader in the field of electrochemical transformation of CO2, and generate long-term economic, social, and environmental benefits nationally and internationally. For Australia that means accelerating progress towards net zero obligations, growing a sustainable economy, and creating jobs for the future.
Led by GETCO2 Director, Prof Xiwang Zhang at the University of Queensland, and together with the Chief Operations Officer, Dr Eloise Larsen, it brings together 74 individuals and 19 universities and partner institutions. ANSTO is affiliated via Dr Bernt Johannessen who is a GETCO2 Associate Investigator.
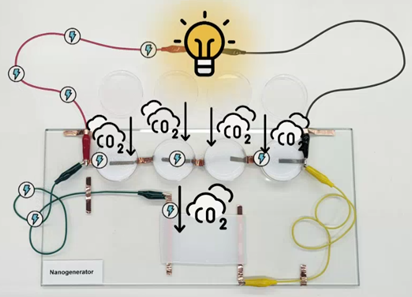
Already this year, Prof Zhang and colleagues developed a carbon-negative nano-generator that consumes CO2 and, in the process, produces electricity. The device has the potential to be scaled up and meaningfully contribute to global efforts towards CO2 reduction.
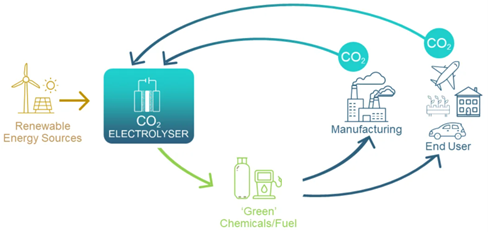
The transformation of carbon dioxide to value-added products will underpin a circular carbon economy in Australia, which helps reshape energy and resource industries. The ability to use renewable energy sources to break the molecular bonds in CO2 gas is key. Then, with the addition of only water and air, desired chemicals such as ethylene, ethanol, and urea, can be formed.
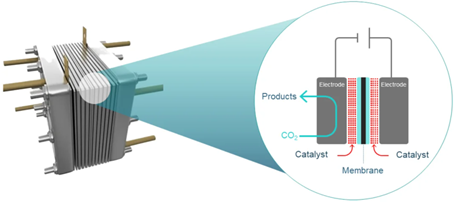
Conversion of CO2 is achieved using an electrolyser comprised of a cathode (negatively charged electrode) and an anode (positively charged electrode) in contact with electrolytes and separated by an ion exchange membrane.
The electrodes are coated with different catalysts to lower the energy required to break chemical bonds in CO2, and to promote selective bond formation to deliver desired products. This CO2 utilisation is expected to be a $1trillion industry by 2030 – within the lifetime of GETCO2.
GETCO2 was launched at the International Symposium on Green Transformation of Carbon Dioxide (ISGTCO2) in Brisbane in December 2023. Dr Bernt Johannessen delivered a keynote address at the event, which was launched by Prof Robin Batterham, GETCO2 Strategic Advisory Committee member and former Chief Scientist of Australia. Presentations covered major topics such as CO2 reduction and capture, catalytic systems, and advanced characterization techniques.
GETCO2 is expected to forge technological breakthroughs to accelerate reaction rates, improve product selectivity, and extend operating life of electrolysers. These are all at the forefront of contemporary challenges currently preventing CO2 electrolysis from reaching commercial viability. The project is divided into four main themes: Electrode & Electrolyser, Catalysts, Membranes and Characterisation & Computation.
The X-ray Absorption Spectroscopy (XAS) Beamline at the Australian Synchrotron is expected to contribute to characterisation investigations.
“The beamline is shaping up to be a substantial contributor to the success of the project, together with several other beamlines,” explained Dr Johannessen.
The XAS technique can determine oxidation states and local atomic structure in a variety of materials, including concentrated solid materials, and dilute, liquid materials, thin films, nanoparticles, single atoms, etc. This capability is essential for studying frontier catalysts. The high-flux XAS Beamline can detect atoms at the parts-per-million (ppm) scale.
Over the past five years, the XAS beamline user community has increasingly focused on Advanced Green Materials, particularly in Catalysis and Energy Storage. Beamtime usage in this area grew from less than 20% then to over 80% now. This shift is exemplified by collaborations such as GETCO2.
“To meet increasing demand for XAS, we have enabled complex in-situ experiments, including catalysis, to be conducted, and more recently implemented new data scanning methods. The latter has improved data collection efficiency to 100% and reduced minimum scan times from minutes to seconds. The benefits are substantial for both our community, facility, GETCO2, and not-to-forget, the future, which is just around the corner!” said Dr Johannessen.

Dr Bernt Johannessen (left) is a Senior Scientist at the XAS Beamline in Melbourne and an Associate Investigator on GETCO2. He is contributing to the characterisation focus led by Prof Karen Wilson (Griffith University) and Prof Debra Bernhardt (University of Queensland).
Together with Prof Zhang, Dr Zeng, and collaborators at the University of Queensland, Bernt is applying for time at the MEX-1 Beamline later 2024 to study single-atom catalytic systems.
Read more about the ANSTO spectroscopy beamlines.
Thanks to Dr Johannessen for his contribution to this article.