
Published on the 28th April 2015 by ANSTO Staff
A new nanoscale molecular platform that was tested at ANSTO's Bragg Institute to recognise proteins from the influenza virus, has the potential to be used in the production of advanced biomedical devices.

Smaller than a cell, nanodevices can operate at the level of crucial biological processes and unlock new and ingenious methods of medical diagnosis, treatment, and prevention that will revolutionise human health.
Bragg Institute scientists are collaborating with researchers from the University of Newcastle upon Tyne and Orla Protein Technologies in the UK, on an approach that involves embedding a biomolecule, a nucleoprotein, on a gold surface coated with a protein scaffold. The biomolecule can detect influenza A or B.
A quick, accurate diagnosis of disease ensures timely treatment but would be highly beneficial in monitoring and managing epidemics. And while some rapid testing devices are available now, the new molecular platform has superior diagnostic attributes.
“The antigen we used is only one molecule thick and is an improvement on current antigen based rapid diagnostic tests,” explained research fellow Dr Anton Le Brun, who has expertise in nuclear techniques. An antigen is a substance that binds to antibodies produced by the immune system.
Researchers chose nucleoprotein for the target molecule because it is produced in large amounts when someone has a flu infection. Because it is not one of the two proteins haemagglutinin or neuraminidase that covers the surface of the influenza virus, it is less susceptible to variation in subtypes which can vary rapidly as the virus evolves.
The measurements were done using ANSTO’s neutron reflectometer, Platypus, which is a leading-edge instrument sensitive enough for analysis at the molecular level.
Neutron reflectometry is a technique used for measuring the thickness and composition of materials on the nanoscale (between 1 to 100 nanometers or 0.000001 mm to 0.0001 mm) and is particularly useful for multi-layered processes at the surface level.
The platform is an improvement on existing antigen-based molecular diagnostic kits that provide a response in the form of a colour change. Approaches based on biomolecular platforms are gaining interest because the results can be read out rapidly on electronic devices and deliver more accurate validations.
The advantage is the enhanced sensitivity and specificity of these platforms to avoid a variation in results caused by the age of the patient or the duration of the illness.
It is expected to reduce the occurrence of both false negatives that can be an issue with existing techniques and reduce false positives.
The sensitivity is on par with reverse-transcriptase polymerase chain reaction (RT-PCR) but the advantage is the speed with which a result is obtained and cost effectiveness.
Le Brun describes the assembly process used to construct the molecular scaffolding platform as ‘relatively straightforward’ once you have the working molecules. The scaffold structure has a 3D shape that is a good indicator of bioactivity.
The molecular platform comprises a series of nano-layers. A base layer of silicon lies under a layer of an iron-nickel alloy that is coated with gold, a protein scaffold molecule and a thiol-lipid filling molecule. Gold is used because it is conductive when coupled to electronic devices and is fairly chemically inert.
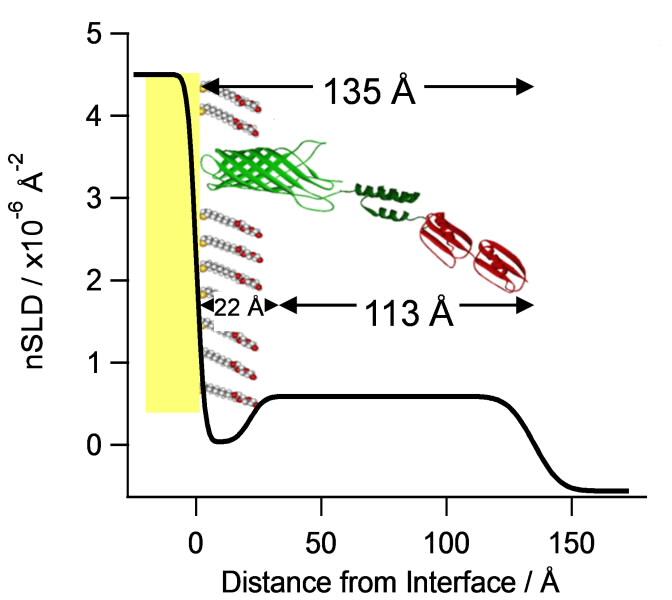
A 13.5 nm thick engineered-monolayer that self-assembles on gold surfaces, captures IgG (antibody) with high affinity in a defined orientation and specifically recognises the influenza A nucleoprotein.
“In the self- assembly process, the molecules spontaneously arrange themselves as a single layer on the gold surface from aqueous solutions under relatively mild conditions,” said Le Brun.
“The molecules that bind to the gold surface have a sulphur-containing thiol functional group that bond to gold. The thiol group has a strong affinity for the gold surface forming a dense single layer one molecule thick of the scaffold protein and the thiol-lipid filling molecule."
After an antibody solution has been added to the membrane-like scaffold, the nucleoprotein will bind specifically to the immobilised antibody. Because the antibodies assemble on the molecular platform in a specific orientation through their affinity for a binding group in the scaffold protein, there is no need for chemical modification or labelling in another step.
This is a system in which the orientation of the antibody is defined. All antigen binding regions of the antibody have the potential to bind antigen as they are orientated to face away from the surface increasing the chance of detection.
The layer of biomolecules has the potential to be assembled onto a microchip. The University of Newcastle upon Tyne has done pioneering work in surface chemistry on proteins and the developments of technologies to detect diseases. Orla Protein Technologies has been developing sophisticated biosensors using electronic chips.
“Neutron reflectometry provides a lot of information about the assembly process, such as deposition temperature, the role of the lipid filling molecule in supporting the structure, the orientation of the bound antibody and the location of the bound nucleoprotein. “
“Neutron reflectrometry is showing us what other techniques cannot because it can measure the thickness and composition of materials on the nanoscale. We are talking about a thickness that is 10,000 times thinner than a sheet of paper,” said Le Brun. “It helps understand how molecules are arranged or assembled in space.”
Biochemistry has become a nanoscience, according to Deepan Shah of Orla Protein Technologies.
“The major player proteins, nucleic acids and membranes all operate on length scales of nanometres and above. Bionanotechnology is in fact a meeting between biomolecules and new technologies that have enabled us to manufacture and measure at the nanoscale…other than benefits of scale, result largely from the novel behaviour of materials at these dimensions.”2 And how would a device depict a positive result?
“Currently we can validates binding with the influenza nucleoprotein by measuring the response with surface plasmon resonance (SPR) or quartz crystal microbalance with dissipation monitoring (QCM-D), said Le Brun “In the future a positive result might be confirmed by something as simple as a flashing light once a value is reached.”
Although the platform that was tested is specific for influenza nanoprotein, it hasn’t been tested on actual clinical samples as yet. Researchers are, however, confident that the scientific evidence to validate the approach has been achieved.
And while the cure for influenza might not happen for many years, the improved detection and diagnosis of it may occur far sooner.
Read the paper here.
1. Salata OV. Journal of Nanobiotechnology [homepage on the Internet]. 2004 [cited 2012 Nov 2].;2(3) Available from: http://www.jnanobiotechnology.com/content/2/1/3
2. D.S. Shah, M.B.Thomas, S. Phillips, D.A. Cosneros, A.P. LeBrun and J.H.Lakey Self-assembling layers created by membrane proteins on gold Biochemical Society Transactions (2007) Vol 35 p522-526