
Enhancing the oxidative stability of squalene
Published by NDF staff 9th December, 2024
The NDF has demonstrated the potential of deuteration for stabilisation of industrially relevant materials. An important proof of concept study showcases how the substitution of hydrogen atoms with the stable isotope deuterium can stabilise key cosmetic and medicinal product components.
It was discovered in 1906 that the liver of a kind of deep-sea shark in Japanese waters contained a large amount of a new hydrocarbon, squalene. Squalene is a versatile, industrially relevant material that is utilised as a dietary supplement (complementing its role in human health – an antioxidant involved in cell stress disease); a vaccine adjuvant (makes the immune system react to a vaccine and induces immunity); a material that is chemically modified for incorporation in drug molecules and biopolymers; and a major cosmetic ingredient for its emollient (skin-softening) properties.
The demand for this ‘functional biomaterial’ is now enormous. Deep water shark livers were the source of this material for the century following discovery, with shark-sourced squalene accounting for about half of production currently. Squalene is now also isolated from plant oils and recent advances have permitted microbial biosynthesis to be employed at scale.
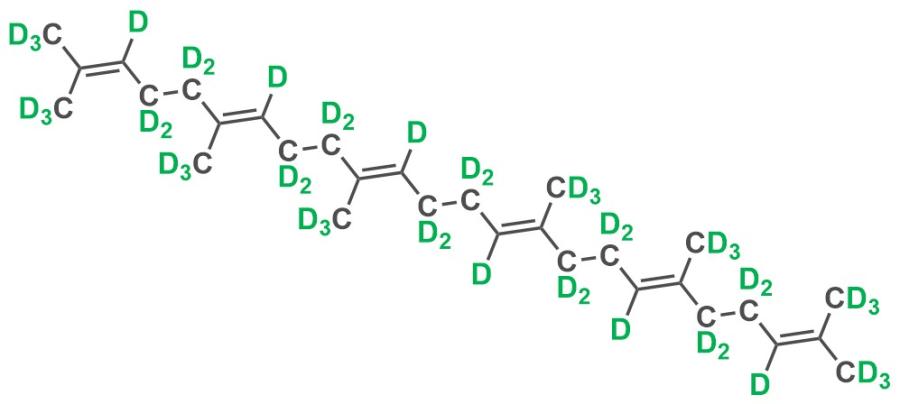
Although a highly useful and utilised material, squalene is susceptible to oxidation and the oxidation products are potentially harmful such as for example, blemish forming (i.e. comedogenic) peroxides on the skin. The National Deuteration Facility at ANSTO utilised microbial production methodology to produce deuterated squalene in order to study this oxidation. Using an engineered yeast (a Saccharomyces cerevisiae strain with high biosynthetic yield of squalene) grown in heavy water, the NDF produced squalene at defined, custom deuteration levels.

The chemical structure of deuterated squalene.
This recent study, published in RSC Advances, showed that the antioxidant activity of the squalene depends on what percentage of hydrogen atoms are replaced by deuterium, with a significant antioxidant effect occurring with levels of deuteration as low as 19%. Cosmetic and pharmaceutical companies can use our results to enhance the performance and stability of formulations.
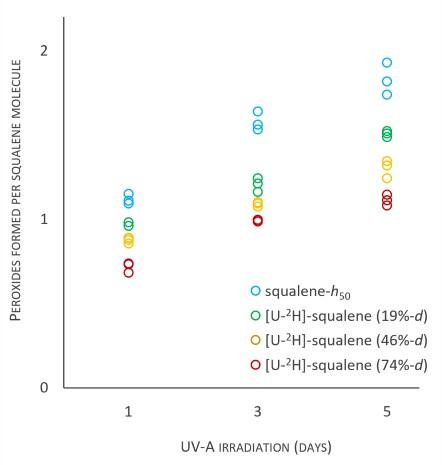
The antioxidant ability increases as a function of deuteration level. This was measured as the number of harmful peroxides formed over time. Light blue = no deuterium, high peroxide level. Red = high deuterium level, low peroxide level. Using UV light mimics exposure of the material to sunlight so we can study its stability as a function of deuterium level.
Reference: Johanna Möller, Carl Recsei, Robert Russell and Tamim Darwish, RSC Adv., 2024, 14, 26002-26006. https://doi.org/10.1039/D4RA05543H
Enquiries
Please direct any enquiries to Tamim Darwish, NDF Leader

Dr Tamim Darwish
The National Deuteration Facility is partly supported by the National Collaborative Research Infrastructure Strategy – an initiative of the Australian Government.
