A large international collaboration from Australia and Europe has used the macromolecular crystallography beamline at the Australian Synchrotron as part of their research in determining the structure of a complex responsible for sorting and delivering cellular cargo.
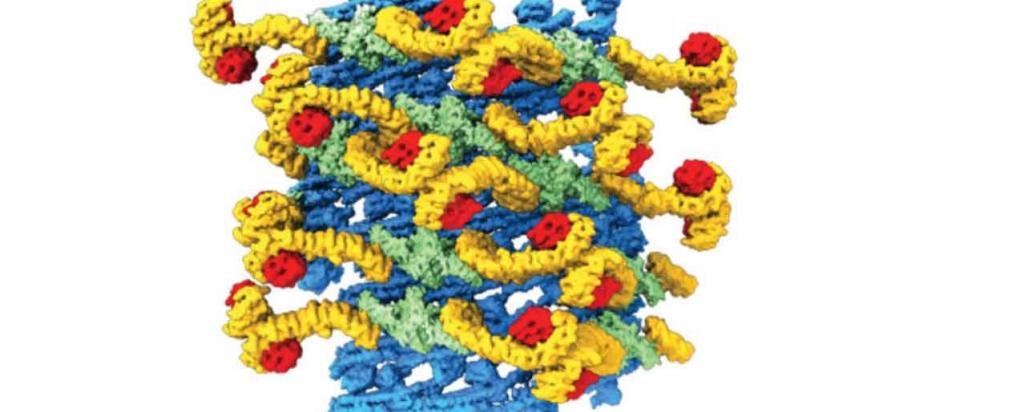
In research published in the esteemed journal Nature, the team used a cutting edge technology, cryo-electron tomography, as the primary method to reveal the three dimensional structure of an entire yeast retromer complex.
Malfunctioning of the retromer complex is thought to contribute to the development of neurological and autoimmune diseases.
The MX1 beamline was used to determine the crystal structure of a component of the complex, Vps29, a protein involved in sorting cellular cargo for transport. Vps29 is comprised of 201 amino acids and forms part of the rigid scaffold residing on the complex.
Principal investigators on the work are associated with the MRC Laboratory of Molecular Biology at Cambridge University, Cambridge Institute for Medical Research (UK) and the Institute of Molecular Bioscience at the University of Queensland with other contributors from the European Molecular Biology Laboratory, and the Max Planck Institute of Biochemistry.
Read more about the research on the Institute of Molecular Bioscience at the University of Queensland website.