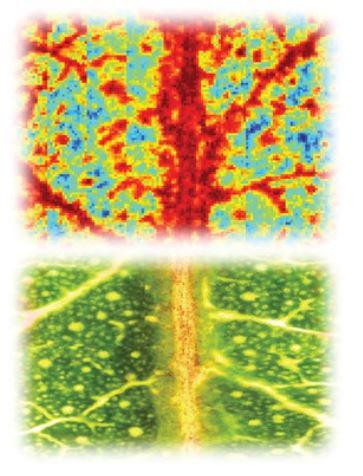
A cross-disciplinary team of Curtin University researchers has used laboratory-based and synchrotron-based infrared spectroscopy imaging techniques to monitor the waxy surface of living plant leaves in real-time to gain insights into plant physiology in response to disease, biological changes or environmental stress.
It is believed to be the first time this has been achieved in living plants and will provide a much-needed new non-destructive analytical method for other agricultural and environmental studies, according to Dr Mark Hackett, an ARC-Future Fellow and analytical chemist at Curtin University with expertise in spectroscopic imaging.
“Such an analytical technique is important because it provides a way to measure the chemical composition of the leaves in a living plant and, moreover, allows us to use this chemical information as a window into plant health,” he added.
A technique known as reflectance Fourier transform infrared spectroscopic imaging (reflectance-FTIR) revealed chemical and physical changes in the wax of both healthy Eucalyptus and wheat leaves infected with yellow spot fungus. The Infrared Microspectroscopy (IRM) beamline at ANSTO's Australian Synchrotron was then used to validate the findings at high spatial resolution using a similar infrared technique, in attenuated total reflectance FTIR (ATR-FTIR) mode.

Fourier transform infrared (FTIR) reflectance spectroscopic imaging has been used to image epicuticular waxes on the surface of plant leaves, in living plants, to observe how plant physiology changes in response to environmental stressors or disease
“This has become increasingly important to understand as crops and plants experience stressors, such as disease, soil acidity, contamination, drought and climate change,” said Hackett, corresponding author on a paper published this week in Advanced Science.
Some analytical techniques alter plant chemistry, which can affect the accuracy of the chemical information to be acquired. Other techniques are also carried out using a section of the plant that has been removed for experimental purposes.
“Although both infrared and Raman spectroscopy don’t require any chemical manipulation when the reflection technique is not applied, they do, unfortunately, still require physical sectioning of the plant,” said Hackett.
“Seeing what is happening in happy, living plants using reflectance FTIR is more ideal,” said Hackett.
The researchers focused on the chemically diverse wax, as it was known to have important protective functions in a plant, such as acting as a barrier, deterring insects and birds that want to feed upon it, protecting against UV and minimising water loss in the plant.
“Infrared techniques can be used because there is a unique infrared chemical fingerprint within the wax layer,” said Dr Mark Tobin, Principal Scientist of the IRM beamline at the Australian Synchrotron and co-author.
Although it was known that the imaging approach might be applicable, because of reflectance that occurs from the surface of thin films, it had not been used on plant wax.
“Infrared radiation does not damage the leaves and has excellent specificity for the composition of the wax,” said Tobin.
And the measurements can be done in an hour or two, without extensive preparation.
Alterations in the composition of the wax and its distribution that are captured in spectroscopic signatures, as seen in this research, may be applicable as markers of plant health.
Having the capability to analyse plants in real-time will enable researchers to study plant disease or environmental stress in detail.
“A single snapshot measurement is really useful but with this reflectance technique we can see how plant physiology changes as a function of time, which is a lot more insightful,” explained Hackett.
In wheat with yellow spot fungus, being able to detect disease by changes in the wax, before it manifests itself on the crop, could be an early detection strategy.
“We were hoping we might be able to detect changes in the leaf, separate from the main disease, perhaps some vulnerability there that allows it spread. And that is what we saw. The waxes were different in regions that were quite separated from the main disease site.”
The fungal infection altered the distribution of the wax.
The approach can be also used to monitor how plants, such as Eucalyptus, respond to seasonal variation and maturation processes.
It was known that the thickness of the wax will vary with the seasons, but the spectroscopic images revealed alterations in the wax during leaf development.
Hackett said the success was due to the strength of the collaboration, which involved crop scientists and analytical chemists from the School of Molecular and Life Sciences and Centre for Crop Disease and Management (CCDM) at Curtin University and synchrotron infrared beamline scientists.
First authors Karina Khambatta and Ashley Hollings of Curtin University were supported by scholarships from the Australian Institute of Nuclear Science and Engineering (AINSE).
Hackett’s Curtin collaborators included Dr Fatima Naim, who has an early career research award from AINSE, Dr Alan Payne, Dr Georgina Sauzier, Lillian Sanglard and Prof Mark Gibberd.
The IRM beamline team at the Australian Synchrotron comprises Dr Mark Tobin, Dr Pimm Vongsvivut and Dr Annaleise Klein.
“Although whole living plants were not used as samples at the Australian Synchrotron, those high-resolution experiments on living leaves confirmed what we were seeing was actually real and corresponded to the plant anatomical structures,“ said Hackett.
“We know the approach works.”
Read more from Curtin University



